Heat Capacity
Heat Capacity: Overview
This topic covers concepts, such as, Ratio of Specific Heats, Molar Specific Heat Capacity at Constant Volume, Relation Between Molar and Principal Specific Heat Capacities & Principal Specific Heat Capacity of a Gas at Constant Pressure etc.
Important Questions on Heat Capacity
For a gas of molecular weight specific heat capacity at constant pressure is
Why the specific heat at a constant pressure is more than that at constant volume.
What is specific heat of gas at constant pressure
At a given temperature, the specific heat of a gas at constant pressure is always greater than its specific heat at constant volume.
and are specific heats at constant pressure and constant volume respectively. It is observed that, for hydrogen gas and for nitrogen gas. The correct relation between and is:
Assertion: The specific heat at constant pressure is greater than the specific heat at constant volume i.e., .
Reason: In case of specific heat at constant volume, the whole of heat supplied is used to raise the temperature of one mole of the gas through while in case of specific of heat at constant pressure, heat is to be supplied not only for heating 1 mole of gas through but also for doing work during expansion of the gas.
An engine takes in moles of air at and and compresses it adiabatically to of the original volume. Assuming air to be a diatomic ideal gas made up of rigid molecules, the change in its internal energy during this process comes out to be . The value of to the nearest integer is
The ratio of translational and rotational kinetic energies for mole gas at temperature is The internal energy of one mole gas at that temperature is Then value of is
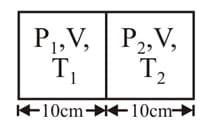
An insulating container of gas has chambers separated by insulating partition. Initially both chambers has equal volume of same gas. Temperature and pressure of left chamber is and and for right chamber is and Now partition is moved towards right by maintaining constant pressure on both sides as earlier and after some time partition is removed without doing any work on gas. If final temperature of gas is find
1 mole of a monoatomic ideal gas and 3 mole of a diatomic ideal gas are mixed. If both gasses can not react then what will be for mixture ? ( and are molar specific heat capacities)
An insulated box containing a diatomic gas of molar mass is moving with velocity . The box is suddenly stopped. The resulting change in temperature is . What will be the value of ?
If and denote the specific heats of nitrogen gas per unit mass at constant pressure and constant volume respectively, then
The ratio of the molar heat capacities of a diatomic gas at constant pressure to that at constant volume is
A molecule of a gas has six degrees of freedom. Then, the molar specific heat of the gas at constant volume is
One gram mole of an ideal gas with the ratio of constant pressure and constant volume specific heats is mixed with gram moles of another ideal gas with . If the for the mixture is , then what will be the value of ?
A rigid triangular molecule consists degrees of freedom. The adiabatic constant of an ideal gas consisting of such molecules is
Specific heat of an ideal gas at constant volume and at constant pressure are related to universal gas constant are as
One mole of an ideal gas is heated at constant pressure through work done by the gas is
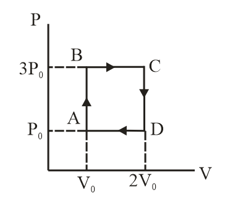
An ideal monatomic gas is carried along the cycle as shown in the figure. The total heat absorbed in this process is
A gaseous mixture consists of of helium and of oxygen. The ratio of the mixture is
